Cue Health COVID‑19 Rapid Molecular Test, Sold by Case (100 Tests | 10 boxes of 10 tests)
Regular price
$5,980.00
Sale
Businesses as well as employees of pre-authorized businesses are permitted to place orders for Cue Health products through Armour.org. Ships to USA and Canada.
Note: The Cue Health Products are exclusively available for sale to verifiable businesses and healthcare providers. Consumers are not permitted to place orders for Cue Health Products through the Armour.org website and any consumer orders for Cue Health Products through the Armour.org website will be cancelled. Armour.org is a division of MM Herman, an authorized distributor of Cue Health products.
"Cue is groundbreaking––it's the first rapid molecular diagnostic test usable by everyone without a doctor or prescription. That means lab-quality results, including for emerging variants, delivered to you in about 20 minutes. No testing lines. No questionable antigen results."
What's Included
One Case of 100 Tests.
Ten 10-Packs of Single-Use Tests, approved for over-the-counter use.
Not Included
Cue Readers are not included but are required for testing. Sold separately here.
Quantity Limits
There is currently no limit on quantity of tests purchased. Orders ship in 3-5 days.
Return / Cancellation Policy
Returns & cancellations are prohibited due to the sensitive nature of the tests.
Bulk Orders
Need more than 500 tests? Order online or contact us.
What is the Cue COVID-19 test?
- Delivers PCR-comparable results in 20 Minutes.
- The Cue COVID-19 test is the first molecular diagnostic test approved for use over-the-counter and healthcare professionals. No prescription or license needed.
-
The Cue COVID-19 Test is chosen by organizations like the NBA and Google to test their people. Cue also recently received a $481 Million award from the United States government.
- Portable for Travel, Education, Healthcare, Government, Corporations, Sports & Entertainment, Remote Employees.
- Made in USA
PCR-Comparable Results in 20 Minutes
-
Gold standard results comparable to PCR delivered straight to you in minutes.
- Cue’s molecular test matched central lab results (PCR) with 97.8% accuracy in an independent study by Mayo Clinic.
-
Cue ranked at the top of the dry swab tests in FDA reference panel, currently with the lowest level of detection (LoD). A lower LoD represents a test's ability to detect a smaller amount of viral material in a given sample, signaling a more sensitive test. (See chart in images.)
-
Both Cue’s COVID-19 test and laboratory tests are molecular nucleic acid amplification tests (NAAT) that detect the SARS-CoV-2 virus genetic material. Molecular tests are more accurate than other methods such as antigen tests.
- The Cue COVID-19 test uses isothermal nucleic acid amplification technology for the molecular amplification reaction that is an equivalent alternative amplification method to polymerase chain reaction (PCR).
Easy & Portable
- The test and reader weigh less than most books and could fit in most purses.
- The test is completely self-contained — no mixing of fluids or complex testing procedure required. No calibration required.
- No training required. Bluetooth reader connects to the Cue app that guides a step-by-step simple testing process. Secure and private results appear in-app.
- If you choose, share or display results for convenient proof-of-test.
Connected & Scalable
- Cue’s molecular test eliminates the need to send samples to a lab, hire healthcare professionals, or perform additional confirmatory tests.
- Quick results and actionable data on location helps save valuable time and prevent larger, costly outbreaks.
- Connect up to six Cue Readers to one mobile device to easily scale up testing when needed.
- For enterprise and professional users, Cue’s API integrations with existing systems enable streamlined health and data management.
What's Inside?
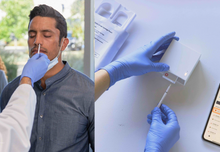
- A single-use Cue COVID-19 Test includes a Cue Sample Wand (to insert into nose) and a COVID-19 Test Cartridge.
-
The Cue Reader is required and sold separately from the tests. Click here to buy just the reader.
-
The HIPAA-compliant Cue Health app is used for step-by-step instructions and reviewing results of each test. Available on iPhone, iPad and Android.
-
Cue’s Cartridges are manufactured in ISO 13485 certified facilities in San Diego, CA using fully automated, highly scalable production lines adhering to Good Manufacturing Practice (GMP) guidelines.
Over-The-Counter Use
- Cue Health received Emergency Use Authorization (“EUA”) for over-the-counter use from the FDA for the company’s easy-to-use, fast, portable COVID-19 test.
- The Cue COVID-19 Test for Home and Over the Counter (OTC) Use (“Cue OTC Test”) is the nation’s first molecular diagnostic test available without a prescription to consumers for home use and to enterprise users and healthcare professionals without CLIA certification.
- The Cue OTC Test is a molecular test that detects the RNA of SARS-CoV-2, the virus that causes COVID-19, using a nasal swab sample taken from the lower part of the nose.
- Results are displayed directly on a connected mobile smart device in about 20 minutes via the Cue Health App.
Do mutations affect the sensitivity of the Cue COVID-19 test?
- Many of the mutations to date have been located in the part of the virus’ genome that codes for spike protein - which is used to gain entry into human cells.
- The Cue COVID-19 test detects a different region of the virus genome that is more stable and conserved. This means the Cue test will likely continue to perform with a high level of sensitivity, even if the virus continues to mutate in the future.
-
At this time, none of the following mutations below affect the sensitivity of the CUE COVID-19 test: Alpha, Beta, Gamma, Delta, Lambda
Shelf Life
- The shelf-life of Cue COVID-19 Test Cartridge Packs are extended from 4 months to 9 months, based on results from Cue’s latest product stability tests.
- All Cue COVID-19 Test Cartridge Packs with a Use By (expiration) date between July 1, 2021, and November 1, 2021, will expire 5 months after the original Use By date printed on the Cue COVID-19 Test Cartridge Pack and the Cue COVID-19 Test Cartridge Pouch labels.
Authorized in USA, EU, Canada, India
-
FDA emergency use authorized for professional use at point-of-care and over-the-counter use without a doctor or prescription.
-
Authorized for professional use in the European Union (CE Mark), Canada (Health Canada Interim Order), and India (CDSCO).
For technical support, call Cue at +1.833.283.8378.